Photocathode Operation and Function - a VERY simplified model.
Do you really want to understand this? Are you sure? If not, click back and go and look at the green pictures. If not read on. If you're a physics expert with a degree in photonics, feel free to email me any criticism or suggestions.
First, to understand photocathodes, you need to understand light. I'll assume you know it's made up of photons. Did you know that different photons have different energies? Given they all travel the same speed, the energy must be something else. In Photon's, it's the wavelength....
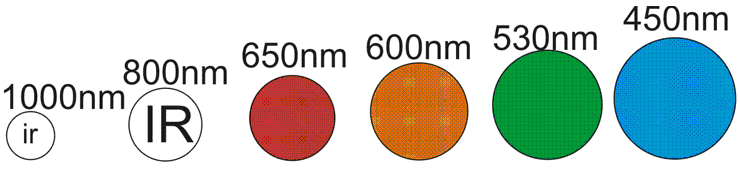
Notice the higher the wavelength, the smaller the size of the "photon" I've drawn? That's no co-incidence. Lower bandwidths have more energy... That's why ultra-violet radiation burns you so badly. It has more energy. You can think of it as a "bigger" photon and we can imagine the size as proportional to the energy. Hence, IR light has less energy than visible light. Easy to understand.
Now, in our photocathode, we have atoms, waiting to accept photons. Clearly they have nothing better to do.
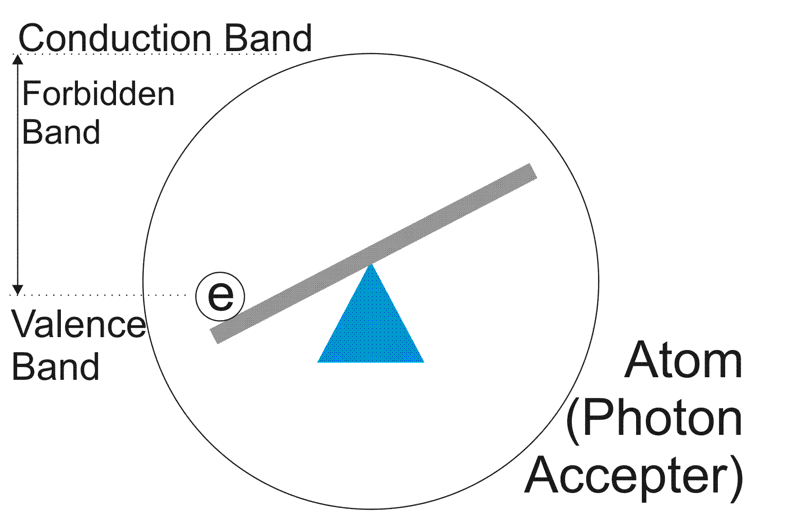
As you can see, if a nicely sized photon smacks into that see-saw, it's going to catapult the electron. If it hits hard enough, it will throw it right outside of the atom, into the conduction band.
Now all photons travel the same speed, but remember the size from the top diagram. A BLUE photon is going to have a lot more impact than a RED photon. In fact, that 1000nm IR photon looks like a weakling. It probably doesn't have enough energy to knock the electron out of the atom at all.
Now if it was a little bigger, say 940nm, and it hits the seesaw right on the end, then it might. And the larger 800nm IR photon could hit the seesaw almost anywhere and catapult that electron out of the atom.
See the effect? This is what affects your photocathode's sensitivity. Above a certain wavelength (higher NM ) the photons are too small to knock the electron out of the atom. On the other hand, more powerful photons like Blue and Green will knock it flying and give it extra kinetic energy.
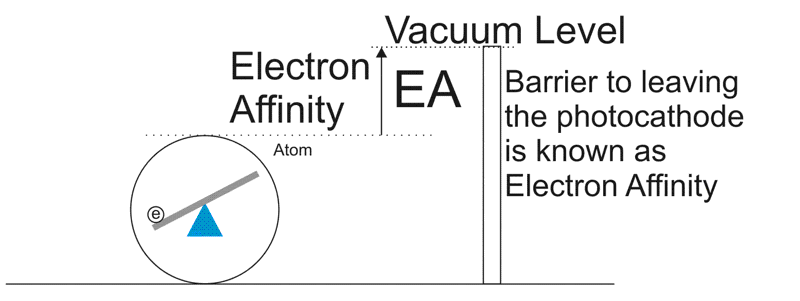
Now you'd think that all you need to do, to knock the electron out of the atom, right? Except the photoconductor doesn't really like losing electrons. It has a surface barrier that causes an Electron Affinity. It's like a fence that the electrons have to get over to make it to the vacuum level. So the electron needs all the extra kinetic energy it gets from the photon to keep going up and over this. Now some atoms are closer to the fence and others are further away. If the atom is too far away from the surface, then the electron might get re-absorbed. That's called Absorbtion loss. Because of this, the optimal thickness of an alkali GenI or GenII photocathode is about 120nm or 1200 Angstrom. Don't ask for the o with the dots. I don't have Unicode on my keyboard.
Also, some photons don't hit any atoms. Ideally, you want to reflect the light back and forth inside the photocathode as much as you can and keep it thin so electrons that do make it into the conduction band don't get lost there... So they coat the photocathode with films like you'd find on lenses ( Those "coated lenses" that have strange colors in reflected light ). This is to try and keep as much light int he photocathode as possible and to improve the efficiency of the photocathode. If the photon doesn't get absorbed on the first pass, maybe it will on the second. That's one of the reasons why different tubes with the same photocathode have different photosensitivities - Some are better at keeping the light in. Amongst other things.
So what else can you do to increase performance?
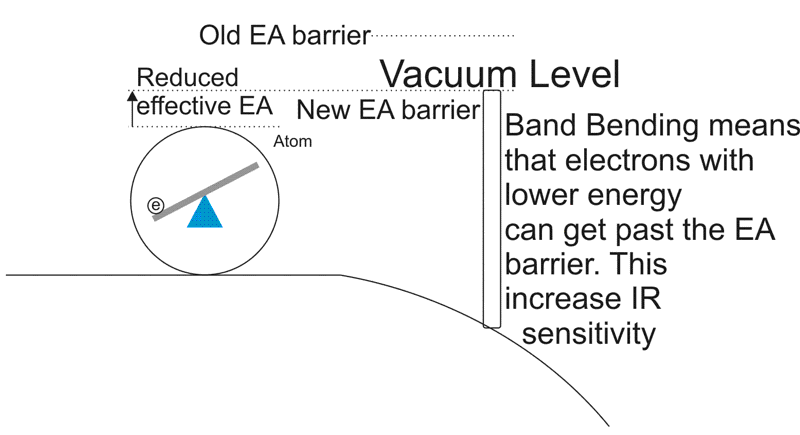
Near the surface barrier, with P-type semiconductor photocathodes, the addition of Cesium, Oxygen and Cesium Oxide ( to name a few ) will reduce the work required to for the electron to make it to the vacuum level.
This means that by adding Cesium and other materials to the photocathode, you can reduce the energy needed. What effect does this have? It increases the sensitivity to longer wavelengths and improves response to Infra-red photons as well as increasing overall sensitivity.
Now, there's one more trick left.

Negative Electron Affinity.
GaAs ( Gallium Arsenide ) is the stuff that scientific wet dreams are made of. It has a lower Electron Affinity ( surface barrier ) than most photocathode materials. In fact, it's so low that once an atom makes it into the conduction band, it can keep on going. That means you can make the stuff REALLY thick so it absorbs all the photons - or at least a LOT more than Generation 2 type photocathodes.
In fact, the optical thickness is about 3um or 3000nm ( 30,000 angstrom ). Compare that to 1200 angstrom for Gen2 photocathodes.
Of course, all that extra photocathode isn't entirely free. It reduces the blue response of the material too, which is why Gen3 tubes aren't all that sensitive to Blue ( while Gen2 tubes are VERY sensitive to blue ).
After that? Then you get into the acceleration of the electron and why Image Tubes needs such high voltages to work ( and what problems it causes ).
-- end of story --
OK, did you like that? Did you understand that? It's an oversimplification but if you get it, it will help with understanding how photocathodes work when you're reading some physics texts on it.